COLLISION RELAPSE

Huidige inclusies:
0/360 (0%)
Doel:
Recurrent Colorectal Liver Metastases: Repeat Local Treatment +/- Neoadjuvant Systemic Therapy - a Phase III Prospective Randomized Controlled Trial
Samenvatting:
After initial treatment of CRLM, new intrahepatic recurrence develops in 64%-85% of patients. To treat recurrent CRLM local treatment is considered standard of care. With upfront repeat local treatment 5-year reaches 51%. Given the poorer prognosis associated with patients with recurrent disease, attributed to presumed worse tumor biology and the presence of intrahepatic micrometastases, neoadjuvant chemotherapy prior to repeat local treatment has been suggested to prolong survival and to select responders who will benefit from local treatment. Although the recommendation of neoadjuvant chemotherapy followed by repeat local treatment is frequently reported, the exact role of neoadjuvant chemotherapy prior to repeat local treatment in case of recurrent and locally treatable CRLM remains uncertain.
Primaire uitkomst:
- Overall survival (OS - intention-to-treat analysis)
Secundaire uitkomsten:
- Distant progression-free survival (DPFS)
- Local tumor progression-free survival (LTPFS) per patient and per tumor treated
- Systemic therapy related toxicity, procedural morbidity and mortality
- Length of hospital stay
- Assessment of pain
- Quality of life (QoL)
- Cost-effectiveness ratio (ICER)
- Quality-adjusted life years (QALY)
Ontwerp:
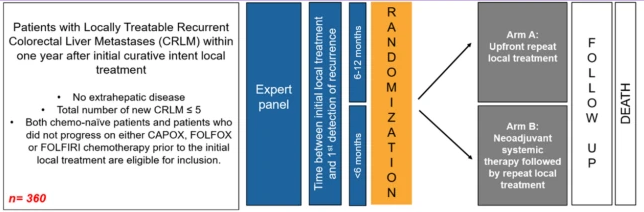
Prospectieve multicenter phase III randomized controlled trial
- Arm A: Upfront repeat local treatment
- Arm B: 12 weeks of neoadjuvant systemic therapy followed by repeat local treatment
Duur:
- 5 jaar follow-up
Inclusiecriteria:
- Histological documentation of primary colorectal tumor
- Local treatment performed for initial CRLM
- >=1 locally treatable recurrent CRLM (partial hepatectomy and/or thermal ablation)
- Resection for resectable lesions considered possible obtaining negative resection margins (R0) and preserving adequate liver reserve
- Total number of new CRLM <= 5
- No microsatellite instability (MSI)
- Good performance status (ECOG 0-2) // ASA 1-3
- No extrahepatic disease
- Both chemo-naïve patients and patients who did not progress on either CAPOX, FOLFOX, or FOLFIRI chemotherapy prior to the initial local treatment
Exclusiecriteria:
- Pregnant or breast-feeding subjects. Women of childbearing potential must have a negative pregnancy test performed within 7 days of the start of treatment
- Immunotherapy <= 6 weeks prior to the randomization
- Chemotherapy <= 6 weeks prior to the randomization
- Progression on both oxaliplatin and irinotecan
- Severe allergy to contrast media not controlled with premedication
Registraties:
| International Clinical Trial Registry Platform (ICTRP) nummer: | CTIS2024-515341-41-01 |
|---|---|
| EU Clinical Trials Register (EUCT): | 2024-515341-41-01 |
| Centrale Commissie Mensgebonden Onderzoek (CCMO): | NL78220.029.22 |